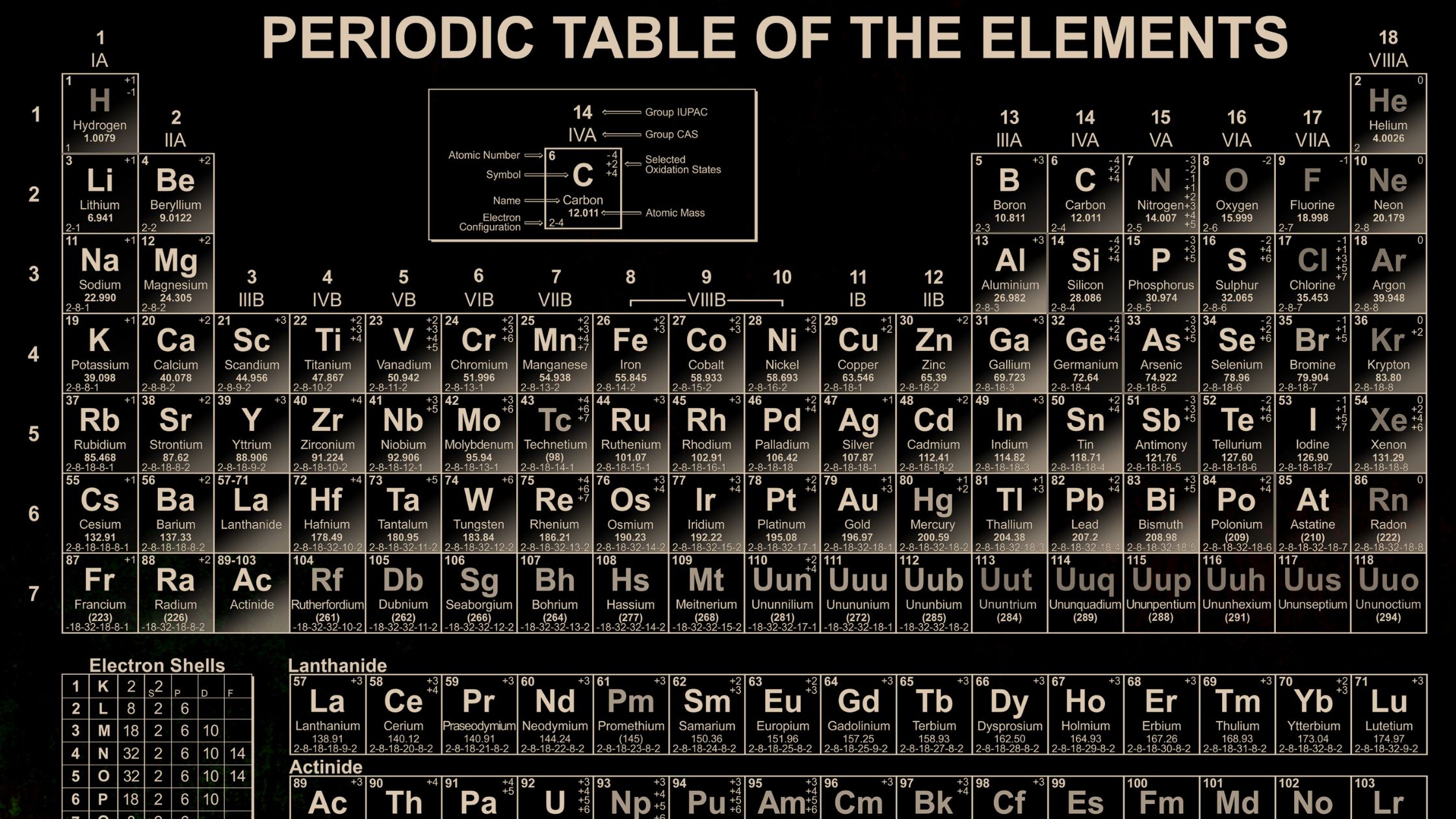
The periodic table is a chart that shows all the chemical elements in an organized way. Here's how it works:
Atomic number: Each element has a unique atomic number, which is the number of protons in its atoms. The atomic number gets bigger as you go from left to right and from top to bottom on the table.
Elements: There are 118 elements on the periodic table. Each element has a symbol made of one or two letters.
Groups: The columns on the periodic table are called groups. Elements in the same group have similar chemical properties. For example, Group 1, called the alkali metals, includes lithium (Li), sodium (Na), and potassium (K), and they all react similarly.
Periods: The rows on the periodic table are called periods. Elements in the same period have similar atomic sizes and behave in similar ways when they form compounds. There are seven periods on the table.
Metals, nonmetals, and metalloids: The periodic table is divided into metals, nonmetals, and metalloids. Metals are good at conducting heat and electricity, nonmetals are not, and metalloids have properties in between metals and nonmetals.
The periodic table helps scientists understand how elements will react with each other to form different compounds.
